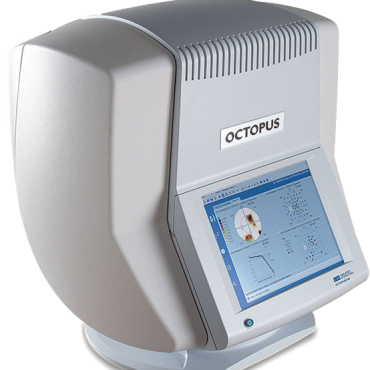
Topcon Henson 9000 Perimeter
Brand: Topcon
SKU: PE0TOHENSON
Price:
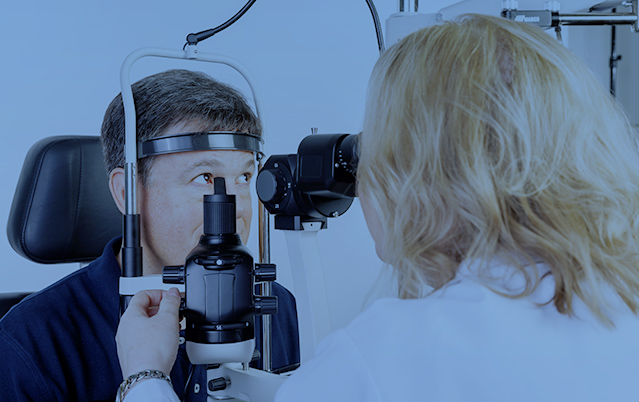
The Topcon Henson 9000 Pro perimeter is compact has a modern design and is easy to use for detecting and managing changes to visual fields.
Free
MSRP:
The Topcon Henson 9000 is a compact, modern and easy to use to use perimeter for detecting and managing changes to visual fields. Featuring innovative analytical tools and time savings tests that enhance patient and staff satisfaction. The 24-2 tests that can be completed in 3 minutes per eye, further enhancing practice efficiencies.
FEATURES
- Speed and Accuracy
- Improved Sensitivity (to Central Defects)
- Convenience
- Enhanced Follow-Up
- Flexible Convenience
- Low Mainenance Perimeter
SPECIFICATIONS
| TEST SPECIFICATIONS | |
| Visual Field Test Range | 60° (monocular) / 160° (binocular) |
| Visual Field Testing Distance | 9.8 in / 25 cm |
| Stimulus Intensity (max) | 10,000 asb |
| Background Illumination | 31.4 asb |
| Stimulus Duration | 200 ms |
| Stimulus Size | Goldman III |
| Stimulus Color | White |
| Test Method | Standard Automated Perimetry (SAP) |
| SCREENING TESTS/PATTERNS | |
| Smart Supra – Single Stimulus | 30, 64 and 86 point tests |
| Smart Supra – Multiple Stimulus | 30, 64 and 86 point tests |
| Esterman (Driving Test) | Groups 1 (120 point) and 2 (124 point) (EU standard) |
| Customized Tests | Test locations can be manually added to all Smart Supra screening tests |
| THRESHOLD TESTS/PATTERNS | |
| ZATA Standard – Threshold Central | 10-48**; 24/30-2 (extendable in-test) |
| ZATA Fast – Threshold Central | 10-48; 24/30-2 (extendable in-test) |
| AVERAGE TEST TIMES | |
| Smart Supra – Single Stimulus | ~1 min (30 points); ~3.5 minutes for fully extended 86 point test |
| Smart Supra – Multiple Stimulus | Under 30 seconds (30 points) |
| ZATA (24-2) | ~3 minutes per eye |
| ZATA Fast (24-2) | ~2.5 minutes per eye |
| FIXATION CONTROL | |
| Fixation Target | Single or 4-point LED diamond pattern |
| Blind Spot Detection | Heijl-Krakau (ZATA) |
| Video Eye Monitor | Yes |
| SOFTWARE FEATURES | |
| Patient Management Database | MS Windows compatible®; networkable |
| Practice Management Integration | EMR compatibility (parameter passing and text file) |
| Hemifield Analysis | Yes |
| Progression Analysis | Yes |
| HFA Data Import | Yes |
| CONNECTIVITY | |
| DICOM | Yes (images) |
| Ethernet | Yes, via connected computer |
| Database Backup | Removable, network or cloud storage |
| DIMENSIONS AND WEIGHT | |
| Dimensions | 17 x 16 x 18 (in) / 440 x 400 x 452 (mm) |
| Weight | 30 lb/ 13.5 kg |
| CLASSIFICATION | |
| Medical Device | Class I |
| Applied Part | Type B |
| Control Device | External PC / laptop / tablet running MS Windows® Professional, v.8, and above |
| Patient Unit Inputs/Outputs | C13 mains input; Patient Response Button; 2 x USB Type B connector |
| Electrical Requirements | 85 – 263 V AC, 50/60 Hz, 60 VA |
| Optional Printer | Any compatible with controlling computer |
Related Products

A Division of Advancing Eyecare™
© 2024 Lombart Healthcare All Rights Reserved.
Account Information
Get to Know Lombart
Lombart Healthcare is committed to keeping our site accessible to everyone. We welcome feedback on ways to improve the site’s accessibility so it is easy for everyone to navigate.
Free shipping is only available for online orders in the continental United States.
Certain exclusions apply.
Exclusions include, but are not limited, to the following products:
Acuity Systems & Projectors, Chair & Stand Accessories, Autorefractors, Lensmeters, Keratometers, Portable Slit Lamps, Stools, Tables, Tonometers, Trial Lens Sets.
- Exam Lane
- Pre-Test
- Diagnostic/Imaging
- Treatment & Surgical
- Lab & Dispensing
- Lens Edgers
- Supplies & Accessories
- Pre-Owned
- All Pre-Owned Equipment
- Pre-Owned Autorefractor/Keratometers
- Pre-Owned Biometers
- Pre-Owned BIOs
- Pre-Owned Chairs & Stands
- Pre-Owned Edgers
- Pre-Owned Lasers
- Pre-Owned Lensmeters
- Pre-Owned Manual Keratometers
- Pre-Owned OCTs
- Pre-Owned Projectors
- Pre-Owned Refractors & Phoroptors
- Pre-Owned Retinal Cameras
- Pre-Owned Slit Lamps
- Pre-Owned Tonometers
- Pre-Owned Ultrasounds
- Pre-Owned Visual Field Perimeters